Zheng Zhixian, Hu Jianqing, Zhu Haijun, Wang Feng, Tu Weiping
(School of Chemistry and Chemical Engineering, South China University of Technology, Guangzhou 510640, China)
Abstract: Polyether diol (GE2000) was used as soft segment and epoxy resin (E-20) was used as partial hard segment to prepare organosilicon cross-linked modified polyurethane emulsion. The effect of silicone content on emulsion properties was discussed. . The results show that as the silicone content increases, the viscosity and molecular weight of the emulsion increase, and the water resistance (solvent), mechanical properties and hydrophobicity of the film are enhanced. FTIR analysis confirmed that the silicone was bonded to the polyurethane structure. SEM analysis showed that the silicone modified polyurethane film was dense. DSC and DMA analysis showed that there was a certain degree of microphase separation between the soft and hard segments of the silicone modified polyurethane, and the increase of the silicone content increased the heat resistance.
Key words: waterborne polyurethane; organosiloxane; modification; composite emulsion
0 preface
Waterborne polyurethane is a binary colloid formed by dispersing polyurethane fine particles in a continuous aqueous phase. It has good mechanical properties, and the temperature of the soft and hard segments is adjustable. It can be cured at room temperature in the fields of coatings, shoes, inks, packaging, construction, etc. Widely used [1-2]. However, due to the presence of its hydrophilic groups, it has defects such as slow drying speed, poor adhesion properties, poor water and heat resistance, and production cost and storage stability [3-5]. Using the characteristics of epoxy resin's rigidity and strong adhesion, the branching point is introduced into the main chain of the polyurethane to form a cross-linked network structure, which can improve its mechanical properties [6]. Silicone has many excellent properties such as low surface energy, high temperature resistance, weather resistance and hydrophobicity [7-.8]. The introduction of organosiloxane into the polyurethane molecular chain to form a block copolymer can improve its water and heat resistance. Adhesive properties, synthetic aqueous copolymers have both excellent mechanical properties of polyurethane, as well as silicone water resistance, thermal stability, surface enrichment, etc. [9-12].
1 test part
1.1 test materials
TDI , polyether glycol (GE2000), dimethylolpropionic acid (DMPA), trimethylolpropane (TMP), epoxy resin (E-20), industrial grade, Shanghai Gaoqiao Petrochemical Third Factory; Ethylamine, ethylenediamine, analytical grade, Guangzhou Jinhuada reagent plant; 1,4-butanediol ( BDO ), analytical grade, Shanghai Lingfeng chemical reagent; aminoethylaminopropyltrimethoxy (A1120), Chemically pure, Compton. 1.2 Synthesis of aqueous dispersion polyurethane-silicone copolymer emulsion GE2000 was added to a four-necked flask equipped with a thermometer, stirring and reflux device, dehydrated at 120 ° C, vacuum 1.33 MPa for 2 h, cooled to 60 ° C, added BDO , heated to 75 After °C, TDI was added dropwise for 2 h, DMPA and E-20 were added for 3 h, the temperature was lowered to 50 ° C, and organosiloxane was added for 1 h. The temperature was lowered to below 40 ° C, neutralized by triethylamine, shear emulsified, and ethylenediamine was added to extend the chain to obtain a silicone-modified aqueous polyurethane dispersion.
1.3 test characterization
1.3.1 emulsion performance test
Viscosity: measured by DV-2+Pro BROOKFIELD viscometer, 1# rotor, rotating speed 60r/min.
Emulsion stability: The appearance of the emulsion was observed and stored at room temperature for 30 d to observe the presence or absence of delamination or precipitation.
1.3.2 film performance test
Water resistance (solvent): A 2 cm × 2 cm film was cut and immersed in water (acetone) at 25 ° C for 24 hours to calculate the water absorption rate (swelling ratio). Tensile strength (elongation at break): A 10 mm × 50 mm film was cut and measured using an Instron Model 5566 electronic tensile machine (Instron, UK) with reference to GB/T 528-1998 at a tensile rate of 200 mm / min.
1.3.3 composite film T-peel strength
The substrate was treated with 0.5% NCS (N-chlorobutene diimide)/acetone solution; the 18 cm×2.5 cm substrate was evenly coated, and after 7 minutes at 70 ° C, the rubber was applied again, and after hot pressing for 5 minutes. Tested by XLL-100 tensile testing machine.
1.3.4 Structure and performance characterization
Molecular weight: determined by HP1100LC-MSD (Hewlett Packard) liquid chromatography mass spectrometer;
Infrared Spectroscopy (FTIR): PerkinElmerspectrum Model 2200 Fourier Infrared Spectrometer (PerkinElmer);
Scanning electron microscopy (SEM): the sample was gold-plated, frozen in liquid nitrogen for 2 h, and analyzed by JSM-5900 electronic scanner;
Differential calorimetry scanning (DSC): using a Netzsch Instruments DSC2910 thermal analyzer, heating rate of 10 ° C / min;
Dynamic mechanical analysis (DMA): using TAinstrument 2980 DMA analyzer, liquid nitrogen protection, temperature -100 ~ 100 ° C, heating rate 2 ° C / min, frequency 110 Hz.
2 results and discussion
2.1 Infrared spectrum analysis
Polyurethane emulsions were prepared by modifying different amounts of A1120 (copolymerization type organosiloxane) (see Table 1).
Table 1 Polyurethane emulsions with different silicone content

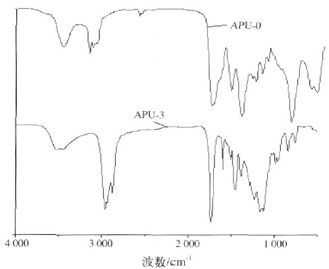
Fig.1 Infrared spectrum of polyurethane emulsion APU-0 and APU-3
The main absorption peaks of the polyurethane in Figure 1 are all in the infrared spectrum, N-H bond stretching vibration peak (3309cm-1), C==O absorption peak (1729cm-1) and amide absorption peak (1537cm-1), C-N stretching vibration peak (1452 cm-1), C-O stretching vibration absorption peak (1231 cm-1), and no asymmetric stretching vibration peak (2270 cm-1) of N==C==O. It can be seen from the spectrum of APU-3 that the introduction of organosiloxane modification resulted in characteristic peaks at Si—O—Si (at 995 cm-1) and Si—C (at 887 cm-1), and at 1109 cm-1. The stretching vibration peak of C-O-C is widened. It can be seen that the organosiloxane A1120 is chemically bonded to the polyurethane.
2.2 Polymer molecular weight and rheological stability
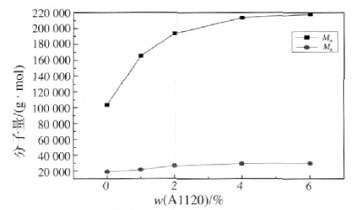
Figure 2 Effect of silicone content on molecular weight of polymer emulsion
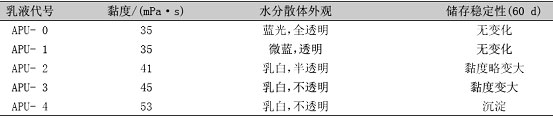
As can be seen in Figure 2, the molecular weight of the polymer in the emulsion increases as the silicone content increases. This is because A1120 belongs to the bisaminosilane coupling agent, and undergoes chain extension reaction with -NCO to crosslink the bridge between the polyurethane molecular chains to increase the molecular weight of the polymer. It can be seen from Table 2 that as the silicone content increases, the viscosity of the emulsion increases, and the appearance and stability deteriorate. This is because after the content of silicone reaches a certain level, the surface migration of the silicone becomes remarkable, and the hydrophilic group is embedded. The strong hydrophobicity of the silicone makes the emulsification effect poor, and the phase separation is severe, resulting in an increase in the viscosity of the emulsion. [13]. When the mass fraction of the organosiloxane is from 2% to 4%, the viscosity and appearance stability of the emulsion are good.
Table 2 Effect of silicone content on emulsion viscosity, appearance and stability
2.3 Mechanical properties of modified polyurethane film
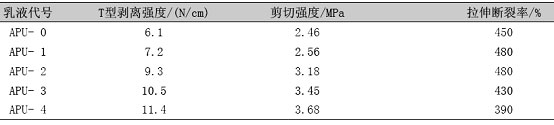
It can be seen from Table 3 that as the content of silicone increases, the T-peel strength and shear strength of the film gradually increase, and the tensile fracture rate first rises and then decreases. The silicone coupling agent is a soft monomer, which has an internal plasticizing effect on the material. When the content of the silicone increases, the tensile strength decreases and the elongation at break becomes larger; the reason for the increase of the T-type peel strength is The silane coupling agent has low surface tension, can spread rapidly on the surface of the substrate, and is in better contact with the substrate. On the other hand, the crosslinking of the silane in the molecular chain of the polyurethane improves the cohesive strength of the aqueous polyurethane adhesive. [14].
Table 3 Effect of silicone content on mechanical properties of film
2.4 SEM analysis (see Figure 3)
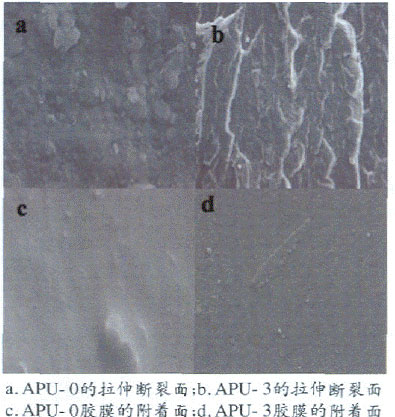
Figure 3 SEM image of APU
In Fig. 3a, the water-based polyurethane film has small cross-section marks and cracks, and there are obvious intact small particles, which is mainly due to the low interaction force between the molecular chains of the polyurethane linear molecules and the low tensile strength; In Fig. 3b, after the film is stretched, no small particles appear on the cross section, which indicates that the introduction of silicone improves the crosslinking degree and tensile strength of the polyurethane. The unmodified polyurethane film attachment surface (Fig. 3c) is relatively loose. The adhesion surface of the silicone-modified polyurethane film (Fig. 3d) is dense and flat, with a slight straight strip pattern. This is because silicone improves the cross-linking degree of the polyurethane chain [15]. The effect shows a high peel strength.
2.5 Water-resistant (solvent) properties of modified polyurethane film (see Figure 4)
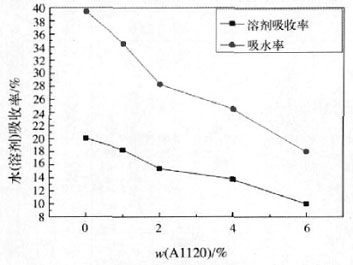
Figure 4 Water-resistant (solvent) properties of modified polyurethane film
As can be seen from Fig. 4, as the silicone content increases, the water absorption (solvent) rate of the emulsion film decreases. This is because the introduction of silane groups causes the surface tension of the polymer to decrease, and the surface energy of the system tends to be minimized, resulting in a component of high surface energy tending to migrate inward, and a component of low surface energy migrates to the surface of the coating film. Thus, the organosiloxane is concentrated on the surface of the emulsion film to improve its water resistance (solvent). The siloxane is hydrolyzed and condensed to form a Si—O—Si bond, which increases the density of the surface layer of the coating film and improves the water resistance of the coating layer. On the other hand, the introduction of the crosslinked organosiloxane A1120 increases the degree of crosslinking of the polymer, so that the interaction between the molecules is enhanced and the solvent resistance is improved [16].
2.6 Surface hydrophobicity of modified polyurethane film (see Figure 5)
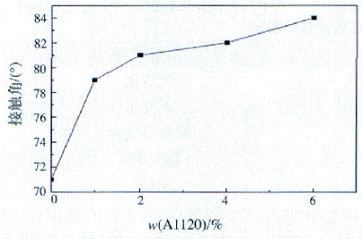
Fig. 5 Effect of silicone content on water contact angle of coating film surface
The contact angle of water on the surface of the coating film was measured using a JC2000A contact angle measuring instrument (Shanghai Zhongchen Company). As can be seen from Fig. 5, as the silicone content increases, the contact angle becomes large. According to the contact angle and surface tension formula:
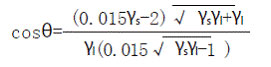
(where γl is the surface tension of water is 72.8mN/m)
From the above formula, it is understood that the surface tension of the polymer decreases as the contact angle θ increases. As the content of silicone increases, the silicone is enriched on the surface of the polymer, the surface polarity is reduced, and the surface tension is correspondingly reduced [17]. The contact angle of water on the surface thereof is increased, that is, the hydrophobicity of the polyurethane coating film is enhanced.
2.7 Thermal Behavior Analysis of Modified Polyurethane
2.7.1 DSC analysis (see Figure 6)
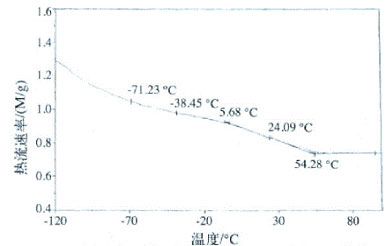
Figure 6 DSC curve of silicone modified polyurethane emulsion APU-3
As can be seen from the DSC curve of Fig. 6, the soft segment Tg of APU-3 was -16.6 °C, and the hard segment Tg was 42.0 °C. Since A1120 contains an amino group, it reacts with -NCO to form a urea group, which increases the hard segment content, and the -Si-O-R segment of the organosiloxane molecule is easily hydrolyzed to form -Si-OH, which crosslinks with -C==O. As the crosslink density increases, the linear molecules crosslink into a network, the ability of the molecular chain to move freely is weakened, and the average chain length between the crosslinks becomes smaller, which increases the rigidity of the hard segment and leads to the water modification of the silicone. Polyurethane has a higher Tg. At the same time, the DSC curve reflects the two glass transition temperatures, indicating that there is microphase separation between the soft and hard segments, and the degree of microphase separation can be further analyzed by dynamic mechanics.
2.7.2 Dynamic Mechanics (DMA) Analysis
The dynamic mechanical properties of polyurethane are closely related to its morphological structure. Due to the difference of the tolerance parameters, there is a degree of phase separation between the hard segment and the soft segment, and the degree of phase separation can be reflected according to the shape and position of the tan δ peak in the dynamic mechanical spectrum [18]. As the compatibility of the two phases increases, the tan δ peak broadens, see Figure 7.
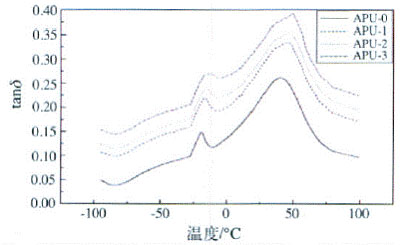
Fig. 7 Relationship between tan δ and temperature of waterborne polyurethane under different contents of silicone
Figure 7 shows a certain degree of microphase separation of the soft and hard segments of the polymer, with two glass transition peaks appearing, and the glass transition of the soft segment appears as a shoulder, which is related to the molecular weight of the soft segment [19]. As the content of silicone increases, the temperature of the β and α internal peaks of the resin increases, and the peaks and peak widths of both internal friction peaks increase. The addition of organosiloxane broadens the internal friction peak of polyurethane, but the temperature at which internal friction begins to increase is maintained at about -20 °C, which has little effect on the low temperature resistance of the resin, but improves the heat resistance of the resin [20]. Highly elastic in a wide temperature range. Therefore, by adjusting the amount of the organosiloxane crosslinking agent, an elastomer having a suitable hardness can be obtained.
3 Conclusion
(1) A silicone cross-linked modified polyurethane emulsion was prepared by using a polyether diol (GE2000) as a soft segment and an epoxy resin (E-20) as a partial hard segment. As the content of silicone increases, the viscosity of the emulsion and the molecular weight of the polymer increase; the water resistance (solvent) of the APU film is enhanced; the T-peel strength and shear strength are increased, and the surface water repellency of the film is enhanced. (2) FTIR analysis showed that the silicone was bonded to the main chain structure of the polyurethane. The SEM image shows that the polyurethane film is more dense after being modified by silicone. (3) DSC analysis showed that the hard segment of the silicone modified waterborne polyurethane was in the glass state and the soft segment was in the high elastic state. DMA analysis showed that there was a certain degree of microphase separation between the soft and hard segments of the silicone modified polyurethane. With the increase of the silicone content, the internal friction peak of the polyurethane became wider, the heat resistance improved, and it was in a wide temperature range. Highly elastic.
Woven Wire Mesh,Chicken Wire Netting,Chicken Wire Fencing
Gabion,Welded Wire Mesh Co., Ltd. , http://www.nsweldedwire.com