Graphene, a single-layer carbon atom honeycomb structure. Due to its unique characteristics of force, heat, light and electricity, it has attractive application prospects in many fields. In 2004, British scientists Andrei Jem and Kostya Novoovov and others first produced graphene by mechanically stripping Graphite blocks by Scoth Tape, and the two worked in 2010. Bell Physics Award. Since then, new methods for preparing graphene have emerged, especially through non-equilibrium surface growth, and it is expected to obtain large-scale high-quality graphene samples.
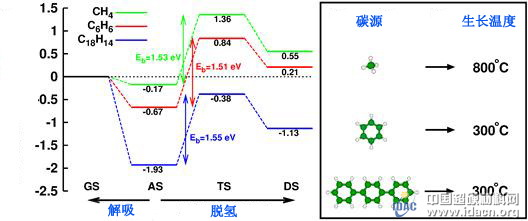
Figure 1. Schematic diagram of the principle of using a different carbon source molecule and corresponding London dispersive force to significantly reduce the epitaxial growth temperature of graphene
The latest research by Dr. Jin-Ho Choi, a postdoctoral researcher of Zhang Zhenyu's research group, and the research team of Professor Zeng Changjun, have better understood the microscopic mechanism of graphene low temperature growth on metal substrates. Choi first theoretically predicts that London's dispersive force (mainly van der Waals force attraction) plays a decisive role in various subtle and competing chemical reactions in the growth of graphene: aromatic molecules have enhanced London dispersive power. Effectively prevents the desorption of adsorbed molecules from the surface and thereby promotes the necessary dehydrogenation reaction. Therefore, low-temperature growth of graphene can be achieved by using a larger aromatic molecule instead of a typical methane as a carbon source (see Figure 1).
The new experiment by Zeng Changjun's research team accurately validated this prediction and further expanded the applicable space of this mechanism. When a new, larger aromatic molecule (p-terphenyl) is used as the carbon source, a large-area high-quality single-layer graphene can be obtained at a temperature of 300 ° C or lower. The Raman spectrum, the light transmission spectrum, and the scanning tunneling microscope image clearly show that the graphene produced is a monolayer thickness. London dispersive force is caused by the ubiquitous transient dipole and is inherently ubiquitous; therefore, this work is of universal importance for the growth of surface nanostructures. The research results were published on May 31, 2013 under the title "Drastic reduction in the growth temperature of graphene on copper via enhanced London dispersion force" (Scientific Reports 3, 1925 (2013)).
The above research work was funded by the National Natural Science Foundation of China, the Chinese Academy of Sciences Foreign Youth Scientist Fund, the Korea Overseas Postdoctoral Fund, and the Ministry of Science and Technology.
Use and performance of graphite electrodes
use
Used in steelmaking electric arc furnace, refining furnace, as conductive electrode; Used for industrial silicon furnace, phosphorus furnace and corundum furnace, as conductive electrode.
performance
High resistance to thermal shock and high mechanical strength
grade
High Power Graphite Electrode (SHP) high power Graphite Electrode (UHP) with high power graphite electrode (HP) with high power graphite electrode (R
Precautions for use of graphite electrodes
1. According to the capacity of the electric furnace and the load of transformer, the grade and diameter of the electrode are suitable
2. When loading the electric furnace, the bulk charge shall be placed in the bottom of the furnace so as not to break the electrode. In the process of smelting, the lime should not be conductive. Large masses of objects are concentrated directly below the electrode, otherwise it will affect the electrode and even cause the electrode to break.
3. Attention should be paid to the position of the furnace cover. If the furnace cover is offset, the cover of the furnace will be blown on the lifting electrode, causing the electrode to be damaged.
4. When connecting the electrode, if the defect of joint bolt is found, the connection should be made after replacement
5. After the electrodes are connected, if there is a gap in the connection surface, it is important to find out the reason
6. The electrode should be used vertically.
7. The electrode holder shall be sandwiched between the upper and lower levels of the top electrode, and shall not be sandwiched between the warning line and the middle electrode. Otherwise, the electrode column is easily broken
8. The raw materials and production techniques of different manufacturers may vary. The physical and chemical properties of the electrode are also different, and it is recommended that the joints and electrodes produced by different manufacturers should not be used in a different way.
High Power Graphite Electrode
High Power Graphite Electrode,Die-Cast Graphite Electrodes,Bone Bit Graphite Electrod,Thermal Graphite
Fengcheng Ruixing Carbon Products Co., Ltd , http://www.lnfcrxts.com